Aerobic and anaerobic respiration of plants. Anaerobic and aerobic respiration
Introduction
1. Aerobic respiration
1.1 Oxidative phosphorylation
2. Anaerobic respiration
2.1 Types of anaerobic respiration
4. List of references
Introduction
Respiration is inherent in all living organisms. It is the oxidative breakdown of organic substances synthesized during photosynthesis, proceeding with the consumption of oxygen and the release of carbon dioxide. A.S. Famintsyn considered photosynthesis and respiration as two successive phases of plant nutrition: photosynthesis prepares carbohydrates, respiration processes them into the structural biomass of the plant, forming reactive substances in the process of stepwise oxidation and releasing the energy necessary for their transformation and vital processes in general. The total breathing equation has the form:
CHO+ 6O→ 6CO+ 6HO + 2875kJ.
From this equation it becomes clear why the rate of gas exchange is used to estimate the intensity of respiration. It was proposed in 1912 by V.I. Palladin, who believed that respiration consists of two phases - anaerobic and aerobic. At the anaerobic stage of respiration, going in the absence of oxygen, glucose is oxidized due to the removal of hydrogen (dehydrogenation), which, according to the scientist, is transferred to the respiratory enzyme. The latter is restored. At the aerobic stage, the respiratory enzyme is regenerated into an oxidative form. V. I. Palladin was the first to show that the oxidation of sugar occurs due to its direct oxidation with atmospheric oxygen, since oxygen does not meet with the carbon of the respiratory substrate, but is associated with its dehydrogenation.
A significant contribution to the study of the essence of oxidative processes and the chemistry of the respiration process was made by both domestic (I.P. Borodin, A.N. Bakh, S.P. Kostychev, V.I. Palladin) and foreign (A.L. Lavoisier, G. Wieland, G. Krebs) researchers.
The life of any organism is inextricably linked with the continuous use of free energy generated by breathing. It is not surprising that the study of the role of respiration in plant life has recently been assigned a central place in plant physiology.
1. Aerobic respiration
Aerobic respiration – This is an oxidative process that consumes oxygen. During respiration, the substrate is decomposed without residue into energy-poor inorganic substances with a high energy yield. Carbohydrates are the most important substrates for respiration. In addition, fats and proteins can be consumed during respiration.
Aerobic respiration includes two main stages:
- oxygen-free, in the process, which is the gradual splitting of the substrate with the release of hydrogen atoms and binding to coenzymes (carriers such as NAD and FAD);
- oxygen, during which there is a further splitting of hydrogen atoms from derivatives of the respiratory substrate and the gradual oxidation of hydrogen atoms as a result of the transfer of their electrons to oxygen.
At the first stage, high-molecular organic substances (polysaccharides, lipids, proteins, nucleic acids, etc.) are first broken down into simpler compounds (glucose, higher carboxylic acids, glycerol, amino acids, nucleotides, etc.) under the action of enzymes. This process occurs in cytoplasm of cells and is accompanied by the release of a small amount of energy, which is dissipated in the form of heat. Further, enzymatic cleavage of simple organic compounds occurs.
An example of such a process is glycolysis - a multi-stage oxygen-free breakdown of glucose. In the reactions of glycolysis, a six-carbon glucose molecule (C) is split into two three-carbon molecules of pyruvic acid (C). In this case, two ATP molecules are formed, and hydrogen atoms are released. The latter attach to the NAD transporter (nicotinamide adenine cleotide), which passes into its reducing form NAD ∙ H + H. NAD is a coenzyme that is similar in structure to NADP. Both of them are derivatives of nicotinic acid, one of the B vitamins. The molecules of both coenzymes are electropositive (they lack one electron) and can play the role of a carrier of both electrons and hydrogen atoms. When a pair of hydrogen atoms is accepted, one of the atoms dissociates into a proton and an electron:
and the second joins NAD or NADP in its entirety:
OVER + H + [H + e] → OVER ∙ H + H.
The free proton is later used for the reverse oxidation of the coenzyme. In total, the glycolysis reaction has the form
CHO + 2ADP + 2HPO + 2 NAD →
2CHO + 2ATP + 2 OVER ∙ H + H + 2 HO
The product of glycolysis - pyruvic acid (CHO) - contains a significant part of the energy, and its further release is carried out in mitochondria. Here, pyruvic acid is completely oxidized to CO and HO. This process can be divided into three main stages:
1) oxidative decarboxylation of pyruvic acid;
2) tricarboxylic acid cycle (Krebs cycle);
3) the final stage of oxidation is the electron transport chain.
In the first stage, pyruvic acid reacts with a substance called coenzyme A, resulting in the formation of acetyl coenzyme a with a high-energy bond. At the same time, a CO molecule (first) and hydrogen atoms are split off from the pyruvic acid molecule, which are stored in the form of NAD ∙ H + H.
The second stage is the Krebs cycle (Fig. 1)
Acetyl-CoA, formed at the previous stage, enters the Krebs cycle. Acetyl-CoA reacts with oxaloacetic acid to form six-carbon citric acid. This reaction requires energy; it is supplied by the high-energy acetyl-CoA bond. At the end of the cycle, oxalo-citric acid is regenerated in its original form. Now it is able to react with the new acetyl-CoA molecule, and the cycle repeats. The total reaction of the cycle can be expressed by the following equation:
acetyl-CoA + 3HO + 3NAD + FAD + ADP + HPO →
CoA + 2CO + 3NAD ∙ H + H + FAD ∙ H + ATP.
Thus, as a result of the decomposition of one molecule of pyruvic acid in the aerobic phase (decarboxylation of PVA and the Krebs cycle), 3CO, 4 NAD ∙ H + H, FAD ∙ H are released. The total reaction of glycolysis, oxidative decarboxylation and the Krebs cycle can be written as follows:
CHO+ 6 HO + 10 NAD + 2FAD →
6CO+ 4ATP + 10 NAD ∙ H + H+ 2FAD ∙ H.
The third stage is the electric transport chain.
Pairs of hydrogen atoms split off from intermediate products in dehydrogenation reactions during glycolysis and in the Krebs cycle are finally oxidized by molecular oxygen to HO with simultaneous phosphorylation of ADP to ATP. This happens when hydrogen, separated from NAD ∙ H and FAD ∙ H, is transferred along the chain of carriers built into the inner membrane of mitochondria. Pairs of hydrogen atoms 2Н can be considered as 2 Н+ 2е. The driving force for the transport of hydrogen atoms in the respiratory chain is the potential difference.
With the help of carriers, hydrogen ions H are transferred from the inside of the membrane to its outside, in other words, from the mitochondrial matrix to the intermembrane space (Fig. 2).
When a pair of electrons is transferred from above to oxygen, they cross the membrane three times, and this process is accompanied by the release of six protons to the outer side of the membrane. On final stage protons are transferred to the inner side of the membrane and accepted by oxygen:
As a result of this transfer of H ions to the outer side of the mitochondrial membrane, their concentration is created in the perimitochondrial space, i.e. an electrochemical gradient of protons occurs.
When the proton gradient reaches a certain value, hydrogen ions from the H-reservoir move through special channels in the membrane, and their energy reserve is used to synthesize ATP. In the matrix, they combine with charged particles of O, and water is formed: 2H + O²ˉ → HO.
1.1 Oxidative phosphorylation
The process of ATP formation as a result of the transfer of H ions through the mitochondrial membrane is called oxidative phosphorylation. It is carried out with the participation of the enzyme ATP synthetase. ATP synthetase molecules are arranged in the form of spherical granules on inside inner membrane of mitochondria.
As a result of the splitting of two molecules of pyruvic acid and the transfer of hydrogen ions through the membrane through special channels, a total of 36 ATP molecules are synthesized (2 molecules in the Krebs cycle and 34 molecules as a result of the transfer of H ions through the membrane).
The overall equation for aerobic respiration can be expressed as follows:
CHO+ O+ 6HO + 38ADP + 38HPO→
6CO+ 12HO + 38ATP
It is quite obvious that aerobic respiration will cease in the absence of oxygen, since it is oxygen that serves as the final hydrogen acceptor. If the cells do not receive enough oxygen, all hydrogen carriers will soon be completely saturated and will not be able to transfer it further. As a result, the main source of energy for the formation of ATP will be blocked.
aerobic respiration oxidation photosynthesis
2. Anaerobic respiration
Anaerobic respiration. Some microorganisms are able to use for the oxidation of organic or inorganic substances not molecular oxygen, but other oxidized compounds, for example, salts of nitric, sulfuric and carbonic acids, which are converted into more reduced compounds. The processes take place under anaerobic conditions, and they are called anaerobic respiration:
2HNO+ 12H → N+ 6HO + 2H
HSO+ 8Н → HS + 4HO
In microorganisms that carry out such respiration, the final electron acceptor will not be oxygen, but inorganic compounds - nitrites, sulfates and carbonates. Thus, the difference between aerobic and anaerobic respiration lies in the nature of the final electron acceptor.
2.1 Types of anaerobic respiration
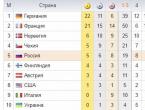
The main types of anaerobic respiration are shown in Table 1. There are also data on the use of Mn, chromates, quinones, etc., by bacteria as electron acceptors.
Table 1 Types of anaerobic respiration in prokaryotes (according to: M.V. Gusev, L.A. Mineeva 1992, with changes)
The ability of organisms to transfer electrons to nitrates, sulfates and carbonates provides a sufficiently complete oxidation of organic or inorganic matter without the use of molecular oxygen and makes it possible to obtain a large amount of energy than during fermentation. With anaerobic respiration, the energy output is only 10% lower. Than with aerobic. Organisms that are characterized by anaerobic respiration have a set of electron transport chain enzymes. But cytochromoxylase in them is replaced by nitrate reductase (when using nitrate as an electron acceptor) or adenyl sulfate reductase (when using sulfate) or other enzymes.
Organisms capable of anaerobic respiration due to nitrates are facultative anaerobes. Organisms that use sulfates in anaerobic respiration are anaerobes.
Conclusion
Organic matter from non-organic green plant forms only in the light. These substances are used by the plant only for nutrition. But plants do more than just feed. They breathe like all living beings. Breathing occurs continuously day and night. All organs of the plant breathe. Plants breathe oxygen and give off carbon dioxide, just like animals and humans.
Plant respiration can occur both in the dark and in the light. This means that in the light two opposite processes take place in the plant. One process is photosynthesis, the other is respiration. During photosynthesis, organic substances are created from inorganic substances and the energy of sunlight is absorbed. During respiration, organic matter is consumed in the plant. And the energy necessary for life is released. Plants take in carbon dioxide and release oxygen during photosynthesis. Together with carbon dioxide, plants in the light absorb oxygen from the surrounding air, which plants need for respiration, but in much smaller quantities than are released during the formation of sugar. Plants take in much more carbon dioxide during photosynthesis than they give off when they breathe it out. Ornamental plants in a room with good lighting emit significantly more oxygen during the day than they absorb it in the dark at night.
Respiration in all living organs of the plant occurs continuously. When breathing stops, the plant, like the animal, dies.
Bibliography
1. Physiology and biochemistry of agricultural plants F50/N.N. Tretyakov, E.I. Koshkin, N.M. Makrushin and others; under. ed. N.N. Tretyakov. – M.; Kolos, 2000 - 640 p.
2. Biology in examination questions and answers L44 / Lemeza N.A., Kamlyuk L.V.; 7th ed. – M.: Iris-press, 2003. – 512 p.
3. Botany: Proc. For 5-6 cells. avg. Shk.-19th ed./Revised. A.N. Sladkov. - M.: Enlightenment, 1987. - 256 p.
Aerobic respiration is the process of releasing the energy contained in organic substances for the life of an organism, in which free oxygen in the air or oxygen dissolved in water is used as an oxidizing agent of substances. Aerobic respiration is carried out by animals and plants, as well as microorganisms.
The emergence of aerobic respiration in the process of evolution.
The oxygen environment is quite aggressive in relation to the microorganism. A moderately strict anaerobic organism survives in an environment with molecular O2, but does not reproduce. Microaerophiles are able to survive and multiply in an environment with a low partial pressure of O2. If an organism is not able to "switch" from anaerobic to aerobic respiration, but does not die in the presence of molecular oxygen, then it belongs to the group of aerotolerant anaerobes. For example, lactic acid and many butyric bacteria.
Obligate anaerobes die in the presence of molecular oxygen O2 - for example, representatives of the genus of bacteria and archaea: Bacteroides, Fusobacterium, Butyrivibrio, Methanobacterium). Such anaerobes constantly live in an oxygen-deprived environment.
Therefore, when the environment of the entire planet many millions of years ago began to accumulate a large amount of molecular oxygen, most of the microorganisms died. Only a small part was able to adapt and start using oxygen for breathing, which gave them a great advantage. And anaerobes remained to develop in the soil and anoxic environments.
7 .3 C BreathSchool:
Date of:
Surname and name of the teacher: Zhakupov AZ
CLASS: 7
Number of people present:
missing:
Lesson topic
Aerobic and anaerobic types of respiration
Lesson type
Combined lesson
Learning objectives that help achieve this lesson
distinguish between anaerobic and aerobic respiration
Lesson Objectives
contribute to the definition and description of aerobic respiration, using the equation of the chemical reaction of the respiration process;
formulate the skills of analysis, generalization when comparing anaerobic and aerobic respiration.
Criteria
evaluation
Students can:
Define and describe aerobic respiration using the equation for the chemical reaction of the respiration process
Compare anaerobic and anaerobic respiration
Language goals
Students can:
describe aerobic and anaerobic respiration verbally and in writing
subject vocabulary and
terminology
aerobic, anaerobic ("an" means without)
(cellular) respiration, glucose, oxygen, carbon dioxide, water, lactic acid, energy
A series of useful phrases for dialogue/writing:
glucose + oxygen → carbon dioxide + water (+ energy)
Instilling value
Ability to work effectively both in a team and individually
Intersubject communications
Chemistry (reaction equations)
Links with ICT
Presentation, use of Internet resources
Previous training
Breathing Grade 6
Plan
Scheduled lesson steps
Planned activity in the lesson
Resources
2 minutes.
7 min
I. Organizing time.
1) Frontal survey of students:
Photosynthesis is
Which plant has photosynthesis
Presentation
15 minutes
10 min
DZ
II. Exploring a new topic
What signs of living organisms do you know?
Nutrition breathing movement irritability reproduction
Breathing in animals
Why do we breathe? How are the process of obtaining energy and breathing related? It turns out that under the influence of oxygen, organic substances break down into simple components: carbon dioxide, water, and sometimes other compounds. During the decay of organic substances, energy is released, which is used by living organisms. They breathe for energy.
As you remember, animals get organic matter from the food they eat. Plants themselves create proteins, fats, carbohydrates, using the energy of light during photosynthesis. From one part of the accumulated organic matter, plants build their own bodies. And the other part of the substances formed during photosynthesis is spent on energy. Plants, like animals, breathe in order to destroy already
created substances and obtain energy from them for life. Fortunately, plants photosynthesize much more than they respire. After all, plants spend almost no energy on the movement of their bodies and work. nervous system and constantly receive it from the Sun (Fig. 66). Therefore, all animals have enough oxygen, which is formed during photosynthesis, and nutrients, with an excess created by plants.
I do it myself writing in a notebook
Breath types.
fill in the table "Comparison of aerobic and anaerobic type of respiration".
Animal drawings
5 minutes
Reflection "Sinkwine"
Name the topic of the lesson in one word
Name 3 things you can do with a theme.
Express in one sentence your impression of the topic of the lesson
What is another name for the theme?
The free energy released during the reaction is stored in the form of a transmembrane proton potential, which is used by ATP synthase to synthesize ATP.
The anaerobic ETC does not contain more pathways for the transfer of protons through the membrane (there are 3 in the aerobic one), and therefore nitrate respiration in terms of efficiency per 1 mol of glucose is only 70% of the aerobic one. When molecular oxygen enters the environment, bacteria switch to normal respiration.
Nitrate respiration occurs, although rarely, among eukaryotes. Thus, nitrate respiration, accompanied by denitrification and the release of molecular nitrogen, was recently discovered in foraminifers. Prior to this, nitrate respiration with the formation of N 2 O was described in fungi. Fusarium And Cylindrocarpon(cm. .
sulfate breath
At present, a number of bacteria are known that are capable of oxidizing organic compounds or molecular hydrogen under anaerobic conditions, using sulfates, inorganic thiosulfates, sulfites, and molecular sulfur as electron acceptors in the respiratory chain. This process is called dissimilation sulfate reduction, and the bacteria that carry out this process are sulfate-reducing or sulfate-reducing.
All sulfate-reducing bacteria are obligate anaerobes.
Sulfate-reducing bacteria obtain energy in the process of sulfate respiration during the transfer of electrons in the electron transport chain. The transfer of electrons from the oxidized substrate along the electron transport chain is accompanied by the appearance of an electrochemical gradient of hydrogen ions, followed by the synthesis of ATP.
The vast majority of bacteria in this group are chemoorganoheterotrophs. The carbon source and electron donor for them are simple organic substances - pyruvate, lactate, succinate, malate, as well as some alcohols. Some sulfate-reducing bacteria have been shown to be capable of chemolithoautotrophy when the substrate to be oxidized is molecular hydrogen.
Sulfate-reducing eubacteria are widely distributed in anaerobic zones of water bodies. different type, in silt, in soils, in the digestive tract of animals. Sulfate recovery is most intense in saline lakes and sea estuaries, where there is almost no water circulation and there are many sulfates. Sulfate-reducing eubacteria play a leading role in the formation of hydrogen sulfide in nature and in the deposition of sulfide minerals. The accumulation of H 2 S in the environment often leads to negative consequences - in water bodies to the death of fish, in soils to the inhibition of plants. Corrosion under anaerobic conditions of various metal equipment, such as metal pipes, is also associated with the activity of sulfate-reducing eubacteria.
fumarate breath
Fumarate can be used as an electron acceptor. Fumarate reductase is similar to nitrite reductase: only instead of a molybdopterin-containing subunit, it contains FAD and a histidine-containing subunit. The transmembrane proton potential is formed in a similar way: proton transfer does not occur, however, fumarate reductase binds protons in the cytoplasm, and dehydrogenases at the beginning of ETC release protons into the periplasm. Electron transfer from dehydrogenases to fumarate reductase usually occurs through the membrane pool of menoquinones.
Fumarate is generally absent from natural habitats and is formed by the microorganisms themselves from aspartate, asparagine, sugars, malate, and citrate. In view of this, most bacteria capable of fumarate respiration contain fumarase, aspartate: ammonia-lyase and asparaginase, the synthesis of which is controlled by the Fnr protein sensitive to molecular oxygen.